An audit is under way to identify Irish children with severe scoliosis who were fitted with a particular type of titanium spinal support rod, which may now need to be removed.
Irish surgeons have been contacted about faults in the Magec System Model X rods, which are implanted in children as young as two years, and are designed to support spinal growth and protect lung development.
The number of Irish patients affected is not yet known, although US manufacturer NuVasive confirmed to The Irish Times that the product has been in use here. They began production in mid-2017.
Children's Health Ireland, the umbrella body for the country's main paediatric hospitals, said it was aware of the recall and is now assessing any potential impact on patients.
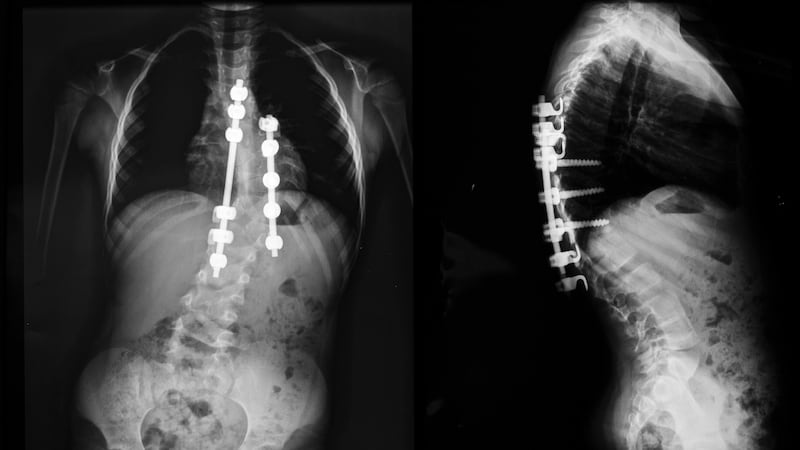
Earlier this month, the rods became the subject of a product recall notice when it was discovered some had experienced the separation of an end-cap in the mechanism, potentially exposing some of its internal components, including alloy debris.
Effects unclear
While NuVasive issued a recall, it said “the decision to remove the device should be made by the physician in consultation with the patient and/or family”.
However, one source familiar with the product said potential effects of any component leakage are unclear.
“There is no advantage to leaving it in and there are many advantages to taking it out,” the source said.
Scoliosis, a severe curvature of the spine, affects about 1 per cent of children and adolescents in Ireland. Treatment options are determined by the severity of the condition and include back bracing and surgery.
Supporting “telescopic” rods of this kind, however, are designed to treat the most severe early onset cases and, crucially, to protect lung development in order to avoid later illness.











